- Products
- Science
-
Resources
- Tinnitus Resources
- What is tinnitus?
- Causes of tinnitus
- Tips for managing tinnitus
- Prepare for your doctor's visit
- PTSD and tinnitus
- Tinnitus FAQs
- Hearing Loss Resources
- What is hearing loss?
- Degrees of hearing Impairment
- Types of hearing loss
- Causes of hearing loss
- Treatment options for hearing loss
- Buy Now
- Coupons
- Test Your Hearing
- Test Your Hearing
- Healthcare Professionals
Our Science
More Than 60 Years of Clinical Experience
Lipo Flavonoid is the leader in ear health supplement innovation and has been for decades. Our natural bioflavonoid products continue to be the #1 recommendation of ENT doctors for relief of ringing in the ears.*
The Vital Parts
of Your Inner Ear
There are five vital components of your inner ear. Lipo Flavonoid supplements provide essential vitamins and nutrients to help them operate at their best.
Click the parts of the ear to explore
Vestibular Labyrinth
Fluid-filled chambers of the inner ear include three tubes called the semicircular canals called the Vestibular Labyrinth. Hair cells in canals detect the motion of the fluid when you move in any direction and convert the motion into electrical signals.
Vestibular Nerve
This nerve transmits the electrical signals from the Vestibula Labyrinth to the brain. This sensory information enables you to maintain your sense of balance.
Auditory Nerve
After the Cochlea converts vibrations into electrical impulses, they are transmitted along the Auditory Nerve to your brainstem via spiral ganglion neurons (SGNs).
Cochlea
The snail-shaped chamber, called the Cochlea, plays a role in hearing. Sound vibrations from the bones of the middle ear are transferred to the fluids of the Cochlea. Tiny sensory hair cells lining the Cochlea convert the vibrations felt through the fluid into electrical impulses that are transmitted along the auditory nerve to your brain.
Auditory or Eustachian Tube
An opening that connects the middle ear with the nasal-sinus cavity to help balance the pressure in the middle ear, drain fluid and protect the ear from sounds and nasal drainage.
Ear Ringing Relief
Learn about the science behind our proprietary Tisina Complex and the other ingredients in the ear ringing and tinnitus relief formula.
Learn more
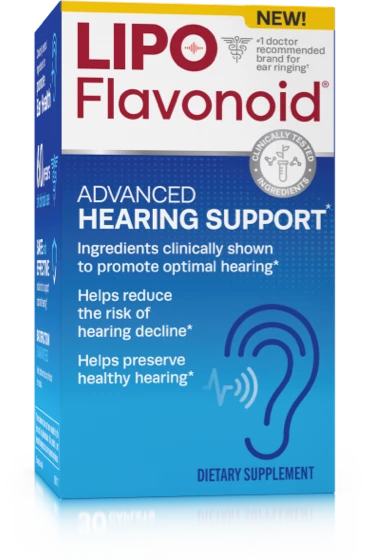
Advanced Hearing Support
Learn about this once daily supplement with an innovative formula full of ingredients clinically proven to help reduce the risk of future hearing decline.
Learn more
Advanced Balance Support
Learn about this daily supplement formulated to help mitigate the risks of dizziness, balance issues, falls, and related declines in quality of life.
Learn more

Proactive Daily Ear Health
Learn about this daily supplement formulated to mitigate the risks of future ear health issues such as loss of hearing, balance issues and ear infections.
Learn more

Trusted by ENTs
According to a recent survey of 250 ear, nose and throat physicians (otolaryngologists or ENTs), 77% of them recommend OTC products/supplements to their tinnitus patients and of them, 74% recommend the brand Lipo Flavonoid.*
Explore our full family of ear health products
Hearing
Support
Support

Balance
Support
Support

Proactive
Ear Health
Ear Health

Ear Ringing
Relief
Relief
Ear Ringing Day/
Night Combo Pack
Night Combo Pack
Ear Ringing
Nighttime Relief
Nighttime Relief
*These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure or prevent any disease.
*Survey data on file
REFERENCES:
- April 2018 Survey. Clarion Brands Inc. data on file.
- Williams H, Hedgecock L. Citrus Bioflavonoids, Ascorbic Acid and Other B-vitamins in the Treatment of certain types of neurosensory deafness a preliminary report. Staff meeting of the Mayo Clinic (1962).
- Tinnitus Overview. Mayo Clinic website http://www.mayoclinic.org/diseases-conditions/tinnitus/basics/definition/con-20021487. Accessed Sept. 7, 2016.
- Understanding the Facts. American Tinnitus Associations website https://www.ata.org/understanding-facts. Accessed Sept. 7, 2016.
- Slattery WH, Fayad JN. Medical treatment of Meniere's disease. Otolaryngologic Clinics of North America 1997; 30:1027-37.
- Kumar S, Pandey AK. Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal. 2013;2013:162750. doi:10.1155/2013/162750.
- Fetterman BL, Saunders JE, Luxford WM. Prognosis and treatment of sudden sensorineural hearing loss. Am J Otol 1996; 17:529-36.
- Arenberg I, Bayer R. Therapeutic Options in Meniere’s Disease. Arch Otolaryngol 1977;103: 589-93.
- Shaia F, Sheehy J. Sudden sensori-neural hearing impairment: a report of 1,220 cases. Laryngoscope 1976; 86:389-98.
- Herschberg S. Meniere’s disease. J Am Osteopathic Association 1974; 73:540-6.
- Wolfson R. Treatment of Meniere’s disease. Modern Treatment (1969) 6,3, 553-567.
- Rubin W. Vestibular suppressant drugs. Arch Otolaryngol 1973; 97:135-8